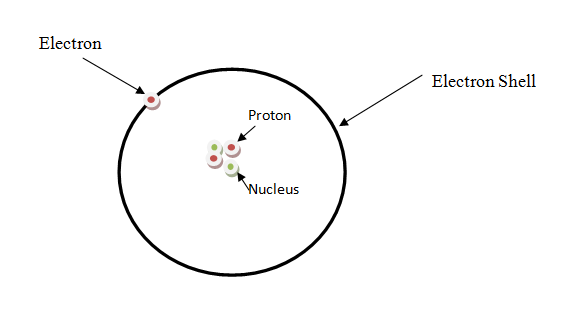
ATOMIC Structure and ATOM
Atom is the smallest particle of an element. It consists of a nucleus, which is composed of neutron and proton, whereas, electrons are found outside the nucleus.
Democritus, a Greek philosopher (460-370 BC) gave an idea that matter is composed of small non-dividing particle, which he named as ‘a-tomio’.
Later, atomic theory given by Dalton in 1808 made the concept more clearer.
DALTON’S ATOMIC THEORY
He stated that:
- Matter is composed of indivisible atoms.
- All the atoms of a given element have identical properties.
- Compounds are formed when atoms of different elements combine in a fixed ratio.
- No new product is formed in a chemical reaction, there is just reorganization.
ATOMIC MASS UNIT
Atomic mass unit can be defined as a mass exactly equal to one twelfth the mass of one carbon-12 atom. It is abbreviated as amu.
1 amu= 1.66056 *10-24g
Mass of an hydrogen atom=1.6736 * 10 g
Mass of H atom in amu= 1.0078 amu
MOLECULAR MASS
Molecular mass is the sum of atomic masses of the elements present in a molecule.
e.g.
Molecular lass of carbon dioxide (CO2)= 12.011u + 2*16.00u = 44.011u
MOLE CONCEPT
One mole is the amount of substance that contain as many particles as there are atoms in exactly 12g of C-12.
One mole of C-12 weighs = 12g/mol
Mass of a C-12 atom = 1.992648 * 10-23 g
The no. of atom in one mole = 6.0221367*1023
AVOGADRO CONSTANT
The number of entities in one mol is known as Avogadro constant. It is denoted by NA.
NA=6.022 * 1023
1 mol of H atoms= 6.022 * 1023 atoms
1 mol of water molecules= 6.022 * 1023 water molecules
Q.1. Which is the smallest particle of an element?
- Compound
- Matter
- Substance
- Atom
Ans. d
Q.2. What are compounds?
- When atoms of different elements combine in a fixed ratio
- When atoms of different elements combine in a non fixed ratio
- Same as atoms
- Smallest particle of an element
Ans. a
Q.3. What is the molecular mass of CO2?
- 33.011u
- 44.011u
- 50u
- 60u
Ans. b
Q.4. 1 mol of H atom is equal to
- 6.022 * 1023 atoms
- 6.032 * 1023 atoms
- 6.022 * 1022 atoms
- 6.022 * 1024 atoms
Ans. a
Q.5. Dalton Atomic Theory states:
- All the atoms of a given element have identical properties.
- Compounds are formed when atoms of different elements combine in a fixed ratio.
- No new product is formed in a chemical reaction, there is just reorganization.
- All
Ans. d
 IT2EDU Empowering Education Through Technology
IT2EDU Empowering Education Through Technology